Introduction: Lymphodepletion (LD) chemotherapy is critical for the efficacy of CAR-T therapy by eradicating immunosuppressive cells, inducing costimulatory molecules, and promoting expansion and persistence of CAR-T cells. It also can contribute to an increased risk of infections, cytopenia, and other toxicities. We evaluated the utilization of varying LD regimens used in standard-of-care (SOC) autologous B-cell maturation antigen-directed (BCMA) CAR-T therapy with ide-cel or cilta-cel for relapsed/refractory multiple myeloma (RRMM) and the effect on outcomes and toxicity.
Methods: Data were retrospectively collected from patients with RRMM who underwent LD and CAR-T cell infusion by December 31, 2022, from 13 US academic institutions with SOC ide-cel or cilta-cel. Response rates and other categorical variables, specifically LD regimen, were tested for association using Wilcoxon rank-sum (or Kruskal-Wallis for three or more groups) or Fisher's exact tests, respectively.
Results: A total of 523 patients were infused at the time of data cut-off with 489 patients evaluable for safety and survival analyses; 368 (75%) were infused with ide-cel and 121 (25%) with cilta-cel. The median age was 65 years old (range 30-90) with 206 females (42%) and 283 males (58%), and 13% with ECOG performance status ≥ 2 at time of LD. Fludarabine and cyclophosphamide (Flu/Cy) LD was used in 422 patients while 67 patients received other LD regimens including bendamustine-based (n = 35, 52%), cladribine-based (n = 25, 37%) or cyclophosphamide alone (n = 7, 10%). The primary reason for use of Flu/Cy LD was institutional guidance or SOC in 378 patients (90%), while the primary reason for use of other LD regimens was due to the fludarabine shortage in 65 patients (97%) with 3% citing physician's choice or institutional guidance.
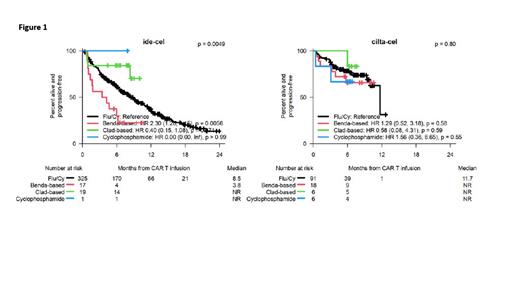
No significant difference was identified among response outcomes, with those receiving Flu/Cy LD achieving an ORR of 84% compared to 81% with other LD regimens (p = 0.59) and no difference observed in rates of ≥ VGPR (66% Flu/Cy LD vs 60% other LD; p = 0.48). Of those evaluable for MRD at 1 and 3 months, the MRD negative rate at 10 -6 for those with Flu/Cy LD was 77% (n= 137) and 77% (n=113) and with other LD regimens was 66% (n=35) and 79% (n=28), respectively. At a median follow-up of 9.7 months, no statistically significant difference was noted in median progression free survival (mPFS) with Flu/Cy vs other LD regimens (9.3 mos vs NR) or overall survival (OS) (19.4 mos vs NR). When stratified by type of CAR-T therapy, treatment with bendamustine-based LD was associated with an inferior mPFS compared to those receiving Flu/Cy LD in patients treated with ide-cel (3.8 vs 8.5 mos; p=0.0056), though not by multivariate analysis. Univariate analysis of patients treated with ide-cel between Flu/Cy and bendamustine LD did not reveal any significant differences in age, gender, ECOG, R-ISS stage, extramedullary disease, high-risk cytogenetics or renal dysfunction (Cr < 45 ml/min). LD regimen was not a predictor for PFS on multivariable analyses amongst patients treated with ide-cel, while ECOG ≥ 3 (hazard ratio [HR]= 4.44, 95% confidence interval [CI]= 1.40, 14.08) and t(4,14) (HR= 2.02, 95% CI=1.14, 3.59) were associated with worse PFS. No difference in mPFS by LD regimen was observed in patients receiving cilta-cel, which was a smaller cohort (mPFS: Flu/Cy 11.7 mos, bendamustine-based NR, cladribine-based NR, cyclophosphamide NR).
Rates of all grade CRS (81% vs 70%), Grade ≥ 3 CRS (3% vs 3%), all grade ICANS (19% vs 12%, and Grade ≥ 3 ICANS (4% vs 2%) were similar among those receiving Flu/Cy or other LD regimens, respectively. Patients receiving Flu/Cy LD were less likely to have any grade cytopenia at 1 month (33% vs 55%, p < 0.001) and 3 months (52% vs 69%, p < 0.001), with no observed difference in infection rate.
Conclusions: The fludarabine shortage experienced in the US in late 2022 necessitated the use of alternative LD regimens with BCMA-directed CAR-T therapy with most centers relying on bendamustine- or cladribine-based regimens. Use of bendamustine-based LD was associated with inferior PFS in patients receiving ide-cel on univariate analysis, but not on multivariate analysis after adjusting for other prognostic factors. In summary, there is no clear difference in efficacy or safety between Flu/Cy and non-Flu/Cy LD in patients receiving BCMA-directed CAR-T therapy.
*Authors contributed equally
Disclosures
Midha:Abbvie: Current equity holder in publicly-traded company; Pfizer: Consultancy. Freeman:ONK Therapeutics: Consultancy, Honoraria; Janssen: Consultancy, Honoraria, Research Funding; Celgene: Consultancy, Honoraria; Bristol Myers Squibb: Consultancy, Honoraria, Research Funding. Castaneda:Adaptive Biotechnologies: Speakers Bureau; Moffitt Cancer Center: Current Employment. Gaballa:Boxer Capital, LLC: Consultancy. Khouri:GPCR Therapeutics: Other: Payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events; Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events. Sborov:GlaxoSmithKline: Consultancy, Research Funding; Janssen: Consultancy, Research Funding; Sanofi: Consultancy, Research Funding; Abbvie: Consultancy; BristolMyerSquibb: Consultancy; Pfizer: Consultancy, Research Funding; Bioline: Consultancy, Research Funding; Arcellx: Consultancy, Research Funding; Gilead: Research Funding; Amgen: Research Funding; Cantex: Research Funding; RocheX: Research Funding. Wagner:Abbvie Inc.: Other: Partner is employed as a medical science liasion. Voorhees:Sanofi: Membership on an entity's Board of Directors or advisory committees; Nervianos Medical Sciences: Research Funding; Regeneron: Consultancy; Karyopharm: Consultancy; Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Oncopeptides: Consultancy; Novartis: Consultancy; GSK: Consultancy, Research Funding; BMS: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Data Safety and Monitoring; Abbvie: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding. McGuirk:Kite: Consultancy, Research Funding; Pluristem Therapeutics: Research Funding; Juno Therapeutics: Consultancy; Allovir: Consultancy, Research Funding; Magenta Therapeutics: Consultancy; EcoR1 Capital: Consultancy; Novartis: Research Funding; Fresenius Biotech: Research Funding; Astellas Pharma: Research Funding; Bellicum Pharmaceuticals: Research Funding; Gamida Cell: Research Funding. Reshef:TScan Therapeutics: Consultancy. Sidana:Magenta Therapeutics, BMS, Janssen, Sanofi, Oncopeptides, Takeda, Pfizer: Consultancy; Magenta Therapeutics, BMS, Allogene, Janssen, Novartis: Research Funding. Hansen:BMS: Consultancy, Research Funding; Janssen: Consultancy; Karyopharm: Consultancy, Research Funding; Pfizer: Consultancy; International Myeloma Society Young Investigator Award: Research Funding; Pentecost Family Myeloma Research Center: Research Funding; Onc Live: Honoraria; Survivorship: Honoraria; BMS IMW Ide-Cel Academic Advisory Board: Membership on an entity's Board of Directors or advisory committees; BMS MM ASH Steering Committee: Membership on an entity's Board of Directors or advisory committees; MM Pfizer Advisory Board: Membership on an entity's Board of Directors or advisory committees. Nadeem:GPCR Therapeutics: Membership on an entity's Board of Directors or advisory committees; Sanofi: Membership on an entity's Board of Directors or advisory committees; BMS: Membership on an entity's Board of Directors or advisory committees; Takeda: Membership on an entity's Board of Directors or advisory committees, Research Funding; GSK: Membership on an entity's Board of Directors or advisory committees; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Patel:Takeda: Consultancy; AbbVie; Allogene Therapeutics, Inc.; Arcellx; Bristol Myers Squibb/Celgene Corporation; Cellectis; Janssen Pharmaceuticals, Inc.; Nektar Therapeutic; Poseida Therapeutics; Precision BioSciences, Inc.; and Takeda Pharmaceuticals U.S.A., Inc.: Research Funding; AbbVie; Arcellx, AstraZeneca; Bristol Myers Squibb/Celgene Corporation; Caribou Science; Cellectis; Curio Bioscience; Genentech; Janssen Pharmaceuticals, Inc.; Karyopharm; Legend Biotech; Merck & Co., Inc.; Oncopeptides; Pfizer; Precision BioSciences: Consultancy.